✔ 100% Authentic Product
👁️ Currently Viewing 2755
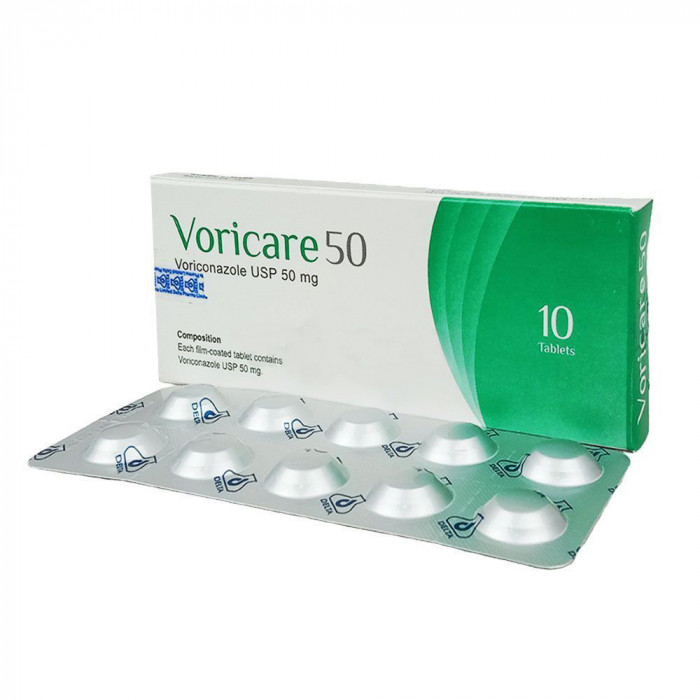
Voricare 50 Tablet 1 Strip
Voriconazole, a triazole antifungal, acts by inhibiting fungal ergosterol synthesis. It specifically targets the CYP450-dependent enzyme 14-α-sterol demethylase, leading to reduced ergosterol in the fungal cell membrane, thereby compromising membrane integrity and exerting its antifungal effect.
Discount
Price: ৳ 190
MRP:
৳
200
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
Voricare is a broad-spectrum triazole antifungal indicated for patients aged 12 years and older for the treatment of:
- Invasive aspergillosis
- Candidemia (in nonneutropenic patients) and deep-seated Candida infections in the skin, abdomen, kidneys, bladder wall, and surgical wounds
- Esophageal candidiasis
- Severe infections caused by Scedosporium apiospermum and Fusarium species, including Fusarium solani
- Patients unresponsive to or intolerant of other antifungal therapies
Use only as directed by a registered physician.
Safety Advices

Alcohol
UNSAFE
It is unsafe to consume alcohol with Voricare 50.

Pregnancy
CAUTION
Voricare 50 is not recommended for use in pregnant women unless considered necessary by the physician. Therefore, please consult your doctor before taking it. Women of childbearing potential must use a suitable contraceptive method to avoid pregnancy while undergoing therapy with Voricare 50.

Breastfeeding
CAUTION
Voricare 50 should be used with caution in breastfeeding women. Consult your doctor before taking it.

Driving
UNSAFE
Do not drive or operate any machines if you experience vision problems after taking Voricare 50.
CONSULT YOUR DOCTOR
Voricare 50 should be used with caution in patients with kidney disease. Consult your doctor before taking it.

CONSULT YOUR DOCTOR
Voricare 50 should be used with caution in patients with liver problems. Consult your doctor before taking it.
✔️ Dosage & Administration of Voricare 50
Oral Use
Take at least 1 hour before or after meals.
≥40 kg body weight
- Loading: 400 mg (10 ml) every 12 hours for the first 24 hours
- Maintenance: 200 mg (5 ml) twice daily
<40 kg body weight
- Loading: 200 mg (5 ml) every 12 hours for the first 24 hours
- Maintenance: 100 mg (2.5 ml) twice daily
Children under 12: Safety and efficacy not established
✔️ Drug Interactions
Enzyme Modulators: Voricare’s efficacy and toxicity may be altered by CYP3A4, CYP2C9, and CYP2C19 inhibitors or inducers. Dose adjustments and close monitoring are advised.
Other Drug Interactions:
May raise levels of CYP3A4, CYP2C9, and CYP2C19 substrates; dose reduction of those drugs may be necessary.
Phenytoin or Efavirenz co-administration may require increased Voricare dosing.
✔️ Contraindications
- Known hypersensitivity to voriconazole or formulation components
- Concurrent use with the following due to risk of serious adverse effects:
- Terfenadine, astemizole, cisapride, pimozide, quinidine, sirolimus
- Rifampin, carbamazepine, long-acting barbiturates, efavirenz, ritonavir, rifabutin, ergot alkaloids, and St. John's Wort
✔️ Side Effects of Voricare 50
Common adverse effects may include:
- Abdominal pain, nausea, diarrhea
- Headache, blurred vision, chest pain
- Anemia
✔️ Use During Pregnancy & Lactation
Voricare should be used during pregnancy only if the potential benefits outweigh the risks, as adequate human data is lacking. Caution is advised during lactation.
✔️ Precautions & Warnings
Long-term therapy (over 180 days) requires risk-benefit reassessment due to potential risks, including squamous cell carcinoma of the skin (SCC).
Monitor liver function and visual changes during prolonged therapy.
✔️ Storage
- Store below 25°C in a dry, light-protected place.
- For powder for suspension: Store between 2°C–8°C prior to reconstitution.
- Keep out of reach of children.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.